Key role of Investigators in patient’s recruitment: Results from MphaR Investigator satisfaction survey

Author: Natalia Denisova, PhD VP, Head of Medical Affairs, MphaR
One of the crucial elements that determine the success of any clinical study is patient enrolment. Patient enrolment or patient recruitment is also one of the most challenging aspects of the study conduct. The problems encountered at the study site include lack of eligible patients, delay in recruitment, and problems with patient retention. McDonald AM et al also noted that in 114 trials, funded by the UK Medical Research Council (MRC) and the Health Technology Assessment (HTA) Programme (recruitment between 1994 and 2002), recruitment was delayed in 47 (41%) trials and early recruitment problems were identified in 77 (63%) trials.1 Furthermore, according to a recently released Tufts CSDD survey, 90% of U.S. trials failed to achieve recruitment targets on time, and 27% of investigator sites are unable to enrol a single qualified patient. Since approximately 29% of a clinical trial’s costs can be attributed to the enrolment process, it imposes significant financial viability on the Sponsor of the study.2 Few studies indicate that one day of delay brings costs to the Company on the level of $600.000 – $8M/day.3 What is more concerning is the fact that 19% of clinical trials were terminated early due to the lack of participants.4
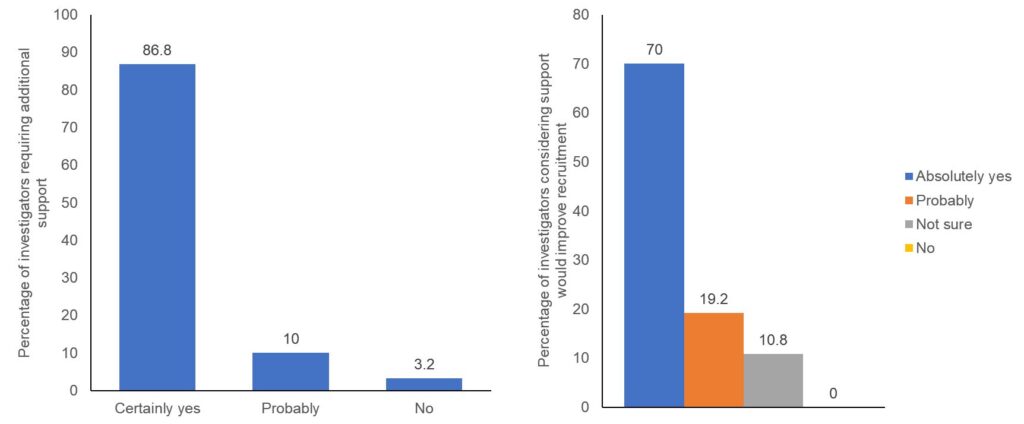
Though patient recruitment is the responsibility of the investigator, the Sponsor should ensure adequate support is provided to the investigator and the study staff during the site initiation as well conduct to ensure that all the personnel involved in the trial are sufficiently trained for patient identification and recruitment. Multiple factors like the study design, backing from the Sponsor, etc have shown to influence the successful recruitment.1 The study team is the link between the Sponsor and the patients, therefore the primary goal of the Sponsor should be to identify and resolve the issues of the study team and the investigator. With an objective to understand the difficulties faced by the investigators in patient recruitment, we surveyed 250 investigators from complex oncology, cardiology, neurology and pulmonology trials across the US, UK, France, and Germany. More than 60% of investigators were involved in 3 or more trials simultaneously and around 89.2% of these felt that they require additional support to understand the complexity of the protocol while participating in the clinical trials. They also said that they had patients enrolment issues for the trials with complex protocol design and were not very satisfied with the quality of interaction with the Sponsor representative (Figure 1). They expected more scientific support from the Sponsor and were particularly keen on having regular interactions
regarding complex protocols to discuss the scientific background, mode of action of the study drug and the study design (Figure 2).
To improve the Sponsor-investigator interaction and facilitate discussion of the protocol, the study design as well as solve study staff query, Investigator Science Liaison (ISL), a new role in the function of Medical Affairs has been introduced. The ISL will enable an additional regular deep scientific discussion that empowers the investigator to understand the study, answers queries and receives feedback. The ISL could also help the Sponsors with the real-time insights from the Investigator, identify protocol challenges, improve under-recruitment, deliver scientific expertise on the current treatment practice and engage early with the leading experts to keep an honest and transparent relationship. Additionally, the ISL would also be able to support the monitors with issue managements, develop trust and confidence in the newly identified investigators and build long-standing collaboration with the regular ones.
Figure 1. Issues faced by the investigators during patient recruitment
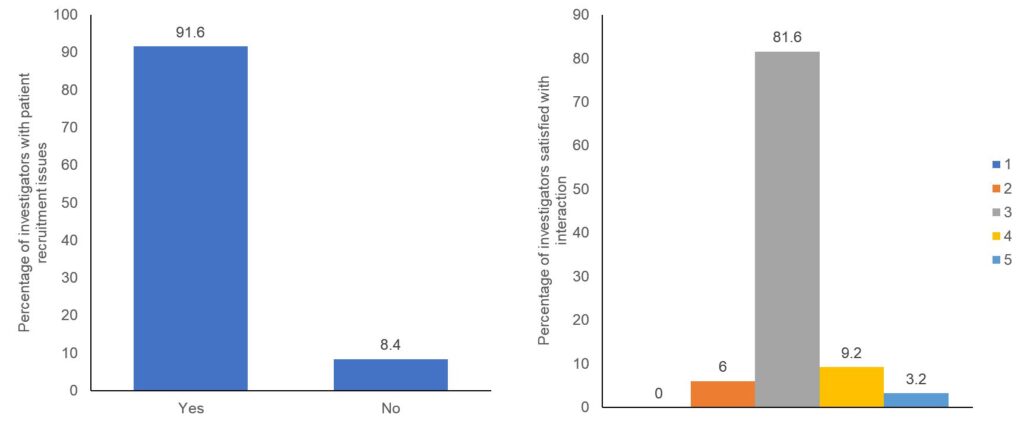
Figure 2: Would ISL intervention improve recruitment?
As the next step of the survey, MphaR solutions engaged ISLs in several complex trials. The investigators were questioned again regarding their satisfaction with the sponsor involvement and the support for the trials. As expected, the patient recruitment was increased by 50% and more than 70% investigators reported being highly satisfied with the introduction of ISL into the study.
Considering the wide scope of responsibilities Investigators Science Liaisons carry and the impact or their work, highly recommend the engagement of ISLs at an early stage of clinical study to help the investigators understand the complexities of protocol and improve patient recruitment.
References
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9.
- https://www.mdconnectinc.com/medical-marketing-insights/tufts-research-barriers-clinical-trial-recruitment-success. Accessed on 09 Jul 2019
- http://www.pharmafile.com/news/511225/clinical-trials-and-their-patients-rising-costs-and-how–stem-loss
- https://www.sciencedirect.com/science/article/pii/S155171441730753X




