Clinical trials in Oncology: challenges with patient recruitment and time to change the status quo

Author: Natalia Denisova, PhD
VP, Head of Medical Affairs, MphaR
Introduction
Despite recent advancements in the field of oncology, cancer remains on the most important public health concerns in developed countries. This group of diseases was responsible for 1.4 million premature deaths in Europe in the year 2018. In the last decade, one out of five people in the EU and the US died due to cancer. The incidence of the disease has raised from 2.1 million to 3.1 million cases between 1995 and 2018 in the EU. Public health experts agree that both population growth and aging are to blame for the increasing annual numbers of newly diagnosed cases of cancer. While more people die because of cancer each year, 5-year survival rates associated with the most common cancer types have improved in Europe and the US between 1995 and 2014 thanks to advances made in patient care which include better screening, diagnostics, treatment and the overall organization of the care process.
In recent years, a record number of new treatment options has become available for oncology patients. New immunotherapies are becoming first-line treatments with biosimilars gaining clinical importance as well. However, despite a promising number of novel cancer therapies that was introduced in recent years, running clinical trials in the field of oncology remains a challenge for pharmaceutical companies. Significant risk of trial failure and long periods needed for new drugs to cross the development pipeline to ultimately reach the market are some of the major obstacles for drug manufacturers. Under recruitment within oncology trials is becoming a significant issue which strips patients of their chance to receive experimental treatment and is a huge waste of the company’s recourses. Each extra day of running a multi-centre clinical trial can cost the company between $600.000 – $8M. In the event of trial cancellation, the financial burden for the sponsor is usually strikingly high.
The clinical trial landscape within oncology is changing at a fast pace as an increasing number of companies actively work to develop new treatment alternatives for patients. There is a shrinking pool of patients who can take part in new clinical trials, therefore as the number of active trials within oncology grows, it is becoming harder to recruit new patients. Clinical investigators are facing multiple challenges. They are often responsible for overseeing several trials that run simultaneously. Modern clinical study protocols for oncology drugs are increasingly complex and often confusing to investigators. In response to a growing demand for on-site scientific support a new role has been introduced within Medical Affairs departments – the Investigator Science Liaison (ISL). Incorporation of ISLs as part of on-site study teams may increase the number of recruited patients by maintaining the investigators scientific interest in the trial and providing guidance through the study protocol.
A shift in the treatment landscape
Since 2004, mortality rates associated with cancer were declining at a steady rate across the EU, US and Japan. According to the 2017 Global Oncology Trends Report the largest decline in mortality was seen among those tumor types where newly approved therapeutics were available and where there were increases in screening and early diagnostics. Out of all cancer types, mortality rates linked to breast, lung and colorectal cancer declined most.
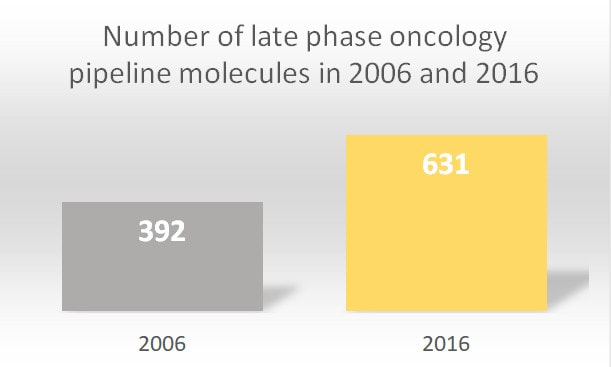
In the year 2018 alone, 15 new therapeutics were launched for 17 separate indications within oncology. Between the years 2014 and 2018, 57 drugs that launched were approved for 89 indications targeting 23 various cancer types. Looking broadly, since 2006, the pipeline of oncology therapeutics has nearly doubled. According to the Global Oncology Trends Report, 87% of drugs in late-stage development included 278 biologic therapies (15 gene therapies, 133 new monoclonal antibodies, 14 biosimilars and 82 vaccines that could target various tumor types). The report underlines that 631 new oncology therapies underwent late-phase trials in the year 2016.
New therapies can now target more tumor types and they are introduced at earlier stages of treatment. In 2018 in the US, more than 200,000 cancer patients were treated with immunotherapies. That was a twofold increase, compared to the year 2016. The use of CDK 4/6 inhibitors in the treatment for HER-2 negative breast cancer has also increased significantly in Europe and the US. More than 50% of the new therapies are delivered orally. Over half of them have an orphan indication.
Additionally, completely new lines of therapies are now available. Those include PD-1, BRAF and MEK inhibitors. They have contributed to an impressive 50% increase in survival and tripled the number of oncology patients receiving medication.
A shift towards personalized medicine can also be observed. In the year 2016, the approach to perform patient segmentation based on their biomarker status was a theme present in 11% of ongoing late-phase trials.
But all this progress comes at a prize. Guidelines governing clinical practice in oncology are transforming because of a record number of new complex therapies. As guideline complexity increases, so does the difficulty with which investigators interpret study protocols.
Unfinished trials and under recruitment
A large proportion of trials is discontinued and ends prematurely which is a big obstacle in the development of promising therapies. Each clinical trial is a complex endeavour that requires sophisticated planning and the mobilization of large amounts of human and financial resources. Therefore, discontinued clinical trials are an incredible waste of effort and money. Each time a clinical study is terminated, ethical and scientific concerns are raised.
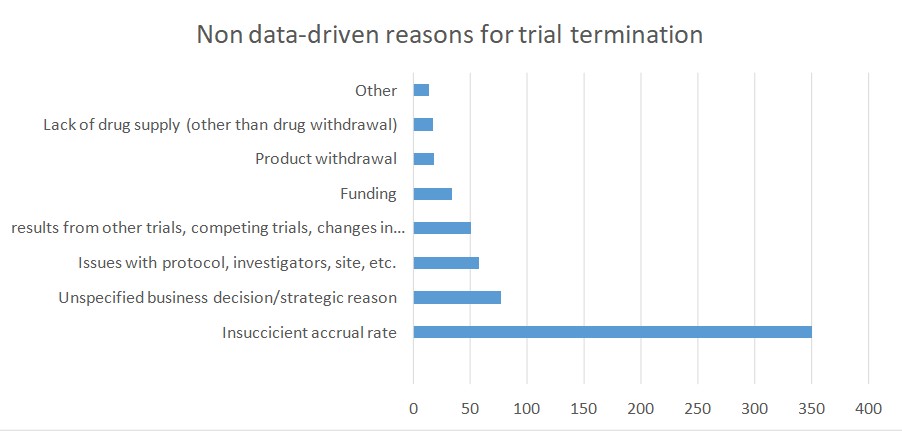
One retrospective analysis of all trials posted on the ClinicalTrials.gov platform showed that 12% of all listed trials were terminated. According to the authors, a great proportion of trials stopped because of a responsible approach that guarantees the ethical conduct of any clinical trial. In many cases, toxicity and efficacy related findings were the reason behind trial termination. That was true especially for clinical trials within oncology, where there is a lack of preclinical models that can serve as a testing ground for new therapeutics.
According to the study, poor patient accrual was another important reason for trial termination. According to a publication by Stendland et all, around 20 percent of all advanced clinical trials within oncology, conducted between 2005 and 2011, was terminated. These trials involved almost 48 000 patient volunteers. Out of 935 trials that were discontinued 39% ended because of poor patient accrual. An additional 20% of trials ended due to various logistical reasons. The downside is that each of the 48 000 patients was exposed to the risk of participation in a clinical trial. Moreover, no advancement in cancer care was made and no societal benefit was achieved. These facts raise ethical issues and should be addressed in future research.
Changing the status quo
It currently costs more than $1 billion to bring a new drug to the market. It was also estimated that each day of delay in running a clinical trial translates to a $600.000 – $8M loss for the company. With financial stakes being so high it is becoming crucial to understand the reasons behind poor patient recruitment within clinical trials.
MphaR’s survey4 among clinical investigators (n=250) who represent the fields of oncology, neurology, cardiology, and respiratory medicine, provides some interesting insights.
- It turns out that most investigators oversee several clinical trials that run simultaneously. More than one-third of investigators taking part in the survey were responsible for recruitment into three to five clinical trials at some point. To successfully recruit patients, the designated clinician must understand the study protocol well. This is not always the case, as study protocols nowadays are proving to be more and more complex.
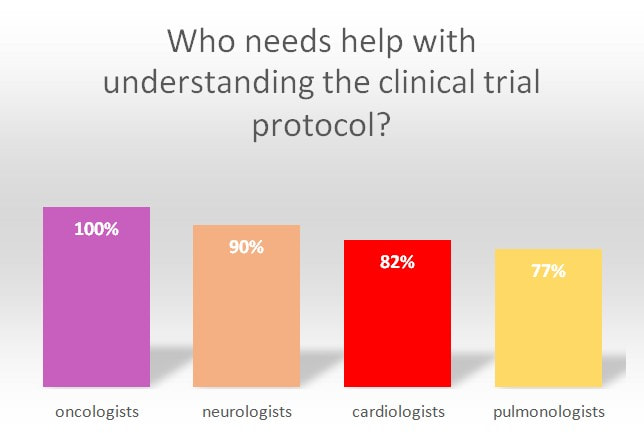
- Nine out of ten clinicians responsible for overseeing clinical trials stated that they would need support with the interpretation of study protocols.
- Interestingly, 100% of oncologists taking part in the survey expressed the need for extra support in this area. Investigators also agree that there is a link between the complexity of study protocols and low patient recruitment. In the survey, more than 90% of investigators stated that study protocol complexity is an issue when it comes to recruiting new patients.
- Most respondents agreed that regular interaction with designated scientific partners during the trial would improve the recruitment process.
- Two out of three clinical investigators agree that such support would speed up patient recruitment. Almost all oncologists who took part in the survey believe that extra support of this kind is needed during a trial.
To face the demanding reality of modern clinical trials a new role has emerged within Medical Affairs departments – the Investigator Science Liaison (ISL). This role is the latest addition to various positions within Medical Affairs and was created in response to the problem of under recruitment within clinical trials.
ILSs regularly interact with investigators and help maintain their interest in the trial by being able to communicate on a deeper scientific level. ISLs look out for any challenge investigators face during a trial. Once a problem is identified, they gather such information and present it to the company. This allows the CRO or pharmaceutical company to come up with solutions that help investigators and in turn improve recruitment.
Additionally, ISLs make sure investigators feel comfortable with the protocol and understand the study objectives well. They also work between monitors and investigators to improve their communication. Any issues reported by clinical monitors can be dealt with on the spot by ISLs which allows for quicker problem solving and better communication in the study team. The input of ISLs does not end here. They contribute to the overall success of the trial by achieving quicker problem-solving at the site and allow for better communication within the study team.
List of references:
- Terminated Trials in the ClinicalTrials.gov Results Database: Evaluation of Availability of Primary Outcome Data and Reasons for Termination (Rebecca J. Williams et al)
- Comparator report on Cancer in Europe – Disease burden, cost and access to medicine (Thomas Hofmarcher et al)
- Global Oncology Trends 2017 – Advances, complexity and cost (IQVIA Institute for Human Data Science)
- CLSA Bulletin, August 1, 2019




